Welcome to the Autumn newsletter!
We have a host of new literature to share in this issue from powder behaviour in 3D Printing to batch variability in Spray Coating. On the video front there’s a new demonstration walkthrough to view and we’d also like to introduce you to our latest recruit, Alex Spinu.
Our next major conference/exhibition is Formnext, maybe see you there…
We hope you’ll enjoy this issue, and as always please do get in touch if you have any questions.
Powder Behaviour in 3D Printing

This App Note describes how the FT4 was able to successfully differentiate three polymeric powders - one containing a pigment, one a lubricant – that performed differently in a Selective Laser Sintering process but were classified as equivalent by traditional powder testing methods.
View App Note here
Controlling the Impact of Humidity

Successfully managing powders in the face of variable humidity is a problem for many processors. ‘Controlling the Impact of Humidity Using the FT4 Powder Rheometer’ explains how powder testing can help and dispels the myth that moisture is always detrimental.
View App Note here
Meeting the Team: Alex Spinu

Alex, who has recently assumed the role of Software Developer, is the latest recruit to the Freeman team. Joining us from Birmingham University with a degree in Robotic Engineering, Alex is already fully immersed in projects to further enhance a feature of the FT4 that we know customers value hugely. Great to have you on board Alex!
New paper - Quantifying Cohesivity

The Spray Coating industry faces exacting demands when it comes to flowability. The data presented in ‘Understanding Batch Variability in Spray Coating Applications’ demonstrate the ability of the FT4 to differentiate polymer powders with a closely similar particle size/particle size distribution, in a process-relevant way.
View App Note here
New paper - Testing for Tableting
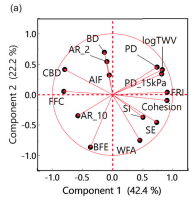
‘Prediction of tablet weight variability from bulk flow properties by sparse modelling’, led by researchers at Osaka Medical and Pharmaceutical University, using the FT4 Powder Rheometer. Dynamic, shear and bulk powder properties all proved significant when it came to reliably describing die-filling performance while at the same time minimising the number of analyses required.
View App Note here
.png)